Usage Protocol
Usage Protocol
- Usage Protocol
- Tips
- Videos
Conversion to MicroFluere® from Traditional Well Plate Using R&D Systems’s DuoSet ELISA Kits
Buffers
The buffers can be used as the same as described in the DuoSet ELISA Kit protocol.
PBS ELISA Plate-coating Buffer catalogue # DY006 from R&D Systems
https://www.rndsystems.com/products/elisa-plate-coating-buffer_dy006
Reagent Diluent Concentrate 2 catalogue Catalog # DY995 from R&D Systems Diluted 10 times in DI water for 1x working concentration.
https://www.rndsystems.com/products/reagent-diluent-concentrate-2_dy995
Quantikine ELISA Wash Buffer 1, Catalog # WA126
Diluted 25 times in DI water for 1x working concentration.
https://www.rndsystems.com/products/quantikine-elisa-wash-buffer-1_wa126
Substrate
MicroFluere® has been tested and compatible with following substrate.
QuantaRed™ Enhanced Chemifluorescent HRP Substrate Kit (Catalog number: 15159
https://www.thermofisher.com/order/catalog/product/15159#/15159
Conversion of working concentrations of reagents for MicroFluere®
A general conversation of reagent concentrations for MicroFluere® is tabulated below.
| Reagent | Concentration- recommended (range) |
| Capture | 2x concentration of traditional well plate (2 to 4x) e.g., 4 µg/mL is used for MicroFluereâ if 2 µg/mL is recommended for traditional well plate. |
| Detection | 1x concentration of traditional well plate (1 to 1.5x) |
| Streptavidin-HRP | 1.5x concentration of traditional well plate (1.2 to 1.8x) |
Example: Human IL-6 (DY206) ELISA Protocol
Stock Solution
First, stock solutions are prepared as described in the product manual (example below).
REAGENT PREPARATION Bring all reagents to room temperature before use. Allow all components to sit for a minimum of 15 minutes with gentle agitation after initial reconstitution. Working dilutions should be prepared and used immediately.
Streptavidin-HRP: Each vial contains 2.0 mL of streptavidin conjugated to horseradish-peroxidase. Dilute to the working concentration specified on the vial label using Reagent Diluent.
Mouse Anti-Human IL-6 Capture Antibody: Refer to the lot-specific C of A for amount supplied. Reconstitute each vial with 0.5 mL of PBS. Dilute in PBS without carrier protein to the working concentration indicated on the C of A.
Biotinylated Goat Anti-Human IL-6 Detection Antibody: Refer to the lot-specific C of A for amount supplied. Reconstitute each vial with 1.0 mL of Reagent Diluent. Dilute in Reagent Diluent to the working concentration indicated on the C of A.
Recombinant Human IL-6 Standard: Refer to the lot-specific C of A for amount supplied. Reconstitute each vial with 0.5 mL of deionized or distilled water.
Working Solution
Second, working solutions are reconstituted based on certificate of analysis (C of A) provided by kit manufacturer to achieve desired concentrations as described in the conversion table.
An example of C of A of Human IL-6 ELISA kit is shown as below.
Certificate of Analysis
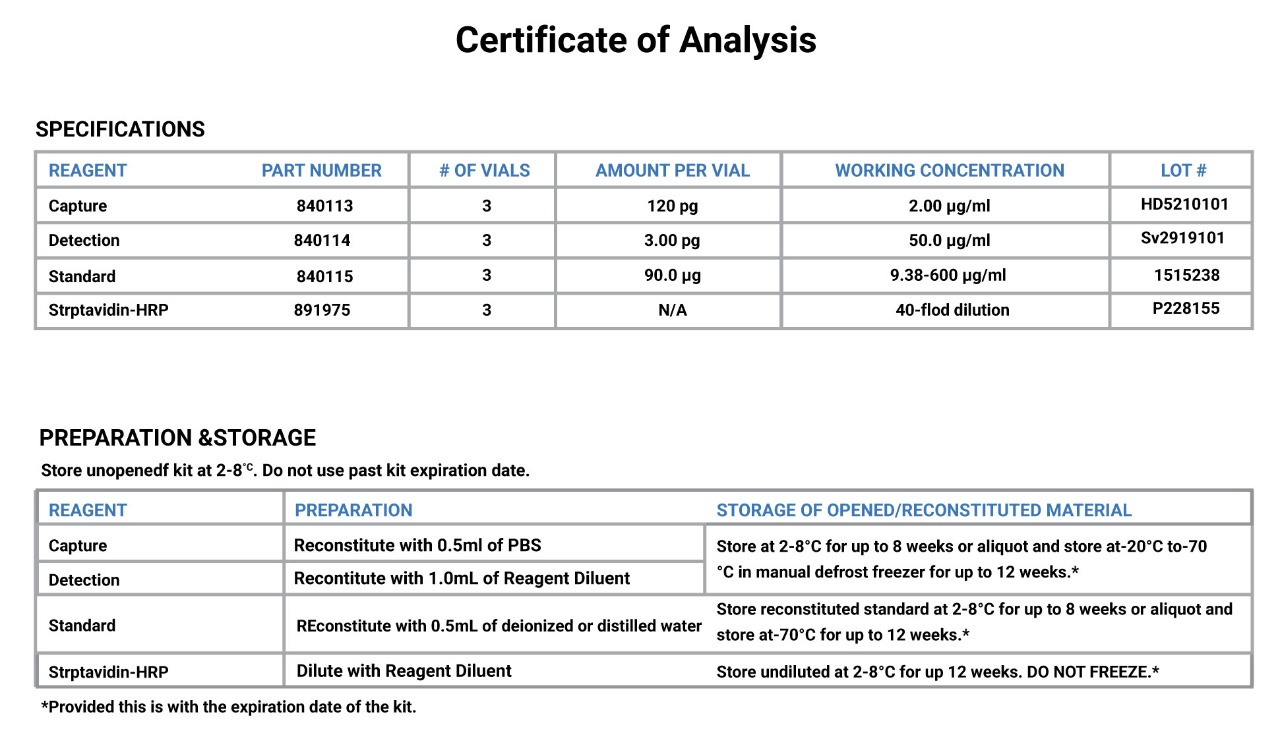
Protocol
| Step | Concentration | Buffer | Volume (µL) | Incubation (min) |
| Capture | 4 µg/ml (60x dilution from stock solution)* | PBS | 20 | 60 |
| Blocking | 1x | 1%BSA | 20 | 30 |
After coating with capture and blocking, the MicroFluere® well plate can be stored in cool dry place for future use, or we can immediately follow following steps.
| Step | Concentration | Buffer | Volume (µL) | Incubation (min) |
| Standard/Sample | Start with 9.6 ng/mL (18.75x dilution from stock solution)*, 2-fold serial dilution until 9.375 pg/mL (11 standards) | 1%BSA | 20 | 15 |
| Detection | 50 ng/ml (60x dilution from stock solution)* | 1%BSA | 20 | 15 |
| HRP | 1:25 dilution | 1%BSA | 15 | 5 |
| Wash 3 times | 1x washing buffer (25x dilution from stock solution)* | 20 | – | |
| Wash 1 time | PBS | 20 | – | |
| Substrate | — | — | 13 | 5-15 |
*Dilution factors as described in the above table are based on the example C of A. The C of A may change lot to lot. Therefore, dilution factors need to be calculated based on recommended working concentrations for MicroFluere®.
Assay Procedure
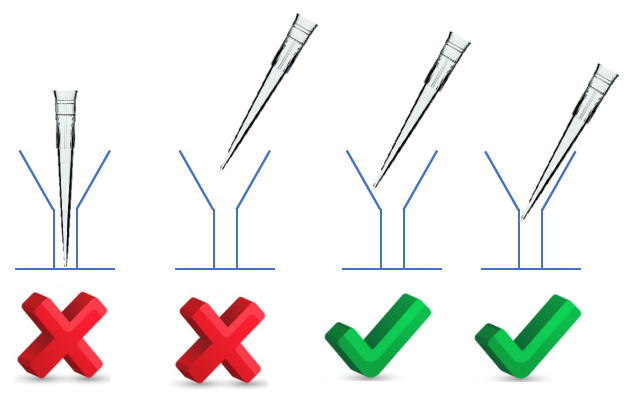
*When mixing antibody working solutions DO NOT vortex/mix them. Only pipette up and down. Vortex is okay for HRP/Substrate* (Please see more detail in liquid handling tips section.)
- Make a working solution of capture antibody; Dispense 20 µL in each well– ensure the liquid flows through the channels; Incubate 60 minutes and then drain the solution using the drainage device
- Dispense 20 µL of blocking buffer in each well; Incubate 30 minutes and then drain the solution using the drainage device
After this step, the plate can be stored in cool and dry place for future use.
- Make standards start with the highest concentration and serial dilutions; Dispense 20 µL in each well; Incubate 15 minutes and then drain the solution using the drainage device
In this step, samples can be loaded together with calibration standards.
- Make a working solution of detection antibody; Dispense 20 µL in each well; Incubate 15 minutes and then drain the solution using the drainage device
- Make a working solution of HRP; dispense 15 µL in each well; Incubate 5 minutes and then drain the solution using the drainage device
- Wash with washing buffer (i.e., dispense 20 µL in each well and then drain the solution using the drainage device), repeat 2 times
- Wash with PBS buffer (i.e., dispense 20 µL in each well and then drain the solution using the drainage device)
- Make working solution of substrate (50:50:1 peroxide: enhancer: ADHP concentrate QuantaRed); Dispense 13 µL/well and incubate covered 5-15 minutes. Do not use stop solution. Do not drain the substrate. The plate is ready to read with a fluorescence microplate reader.
- Read with a Fluorescence microplate reader ( ~550 nm excitation and 605 nm emission wavelengths)
Insert a new absorbent pad and closes the lid. Wait for ~30 seconds before pressing the pump three to five times. Wait for ~20 seconds, then remove the plate. (Please see more detail in the Drainage Device video.)
Sample preparations note:Sample can be diluted in 1x of Reagent Diluent Concentrate 2 (1% BSA) with desired dilution.
Tips for ELISA with MicroFluere Plates
1.Before starting, make sure the environment is free of any fans that could cause evaporation. After every step make sure to put plates in a sealed container or drawer or box.